What are Acid Sulfate Soils and Why are They a Problem?
Acid sulfate soils are those soils in which sulfuric acid (H2SO4) may be produced, is being produced, or has been produced in amounts that have a lasting effect on the main soil characteristics (Prof. Leon Pons 1973).
Acid sulfate soils occur naturally in both coastal and inland settings where waterlogged conditions with sufficient organic matter, iron and sulfate is, or has previously been, present.
These conditions are suitable for the formation of reduced iron sulfides such as pyrite (FeS2) and iron monosulfide (FeS), the active "ingredient" in acid sulfate soils.
Left undisturbed, these soils are harmless, but when excavated or drained, the reduced iron sulfide minerals can oxidise and the soil can acidify (pH<4) due to the formation of sulfuric acid. Once acidified, acid sulfate soils pose a significant environmental risk, due to the low pH of the soil and leaching and transport of the acidity into groundwater or surface water systems.
The consequences following soil and water acidification are typically very severe and include:
- Lethal and sub-lethal effects fish and other aquatic fauna
- Release of dissolved metals and metalloids such as aluminium and arsenic impacting on water quality and water supplies
- Plant death (loss of agricultural production, ecosystem damage)
- Infrastructure damage (roads, bridges, houses)
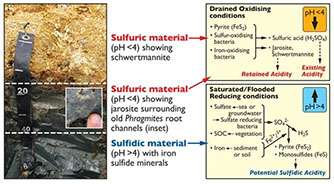
Key biogeochemical reactions in acid sulfate soils are shown in the diagram below and are linked to drying (oxidation) and wetting (reduction) cycles. The redox boundary often determines the depth to which soil oxidation and acidification (to form sulfuric material) will occur with reduced, non-acidic soil (sulfidic material) below this boundary.
Secondary minerals such as Jarosite and Schwertmannite often form in the oxidised acidic layer, and in oxidised surface waters, and along with low pH are a useful visual indicator that acid sulfate soils are present.